Archive: 2026/01
Stability Testing Requirements: Temperature and Time Conditions for Pharmaceutical Products
Stability testing ensures pharmaceutical products remain safe and effective over time under specific temperature and humidity conditions. ICH Q1A(R2) sets global standards for long-term, accelerated, and intermediate testing with precise time and environmental requirements.
Read moreMedications and Work Safety: How Prescription Drugs and Hazardous Drugs Impact Job Performance and Workplace Risk
Prescription medications like opioids and benzodiazepines impair worker safety, while hazardous drugs like chemotherapy agents expose healthcare workers to cancer and reproductive risks. Learn how exposure happens, what works to prevent it, and why current protections fall short.
Read moreHow to Measure Children’s Medication Doses Correctly at Home
Learn how to safely measure children's medication doses at home using milliliters and oral syringes. Avoid common errors that can lead to under- or overdosing, and discover the tools and techniques trusted by pediatricians.
Read moreSerious vs Non-Serious Adverse Events: When to Report in Clinical Trials
Learn the critical difference between serious and non-serious adverse events in clinical trials. Understand when to report, why timing matters, and how to avoid common mistakes that waste resources and risk patient safety.
Read moreUsing One Pharmacy for Better Medication Safety: How Coordination Prevents Dangerous Interactions
Using one pharmacy for all your prescriptions reduces the risk of dangerous drug interactions, prevents duplicate medications, and improves adherence through medication synchronization. It’s a simple step that saves lives.
Read moreTherapeutic Equivalence: Are Authorized Generics Really the Same as Brand Drugs?
Authorized generics are identical to brand-name drugs in every way - same ingredients, same manufacturer, same quality. Learn how they compare, why they’re not listed in the Orange Book, and when they’re the best choice for patients.
Read moreHow to Appeal Insurance Denials for Brand-Name Medications
Learn how to successfully appeal an insurance denial for brand-name medications with step-by-step guidance, critical documentation tips, and real success rates from internal and external reviews.
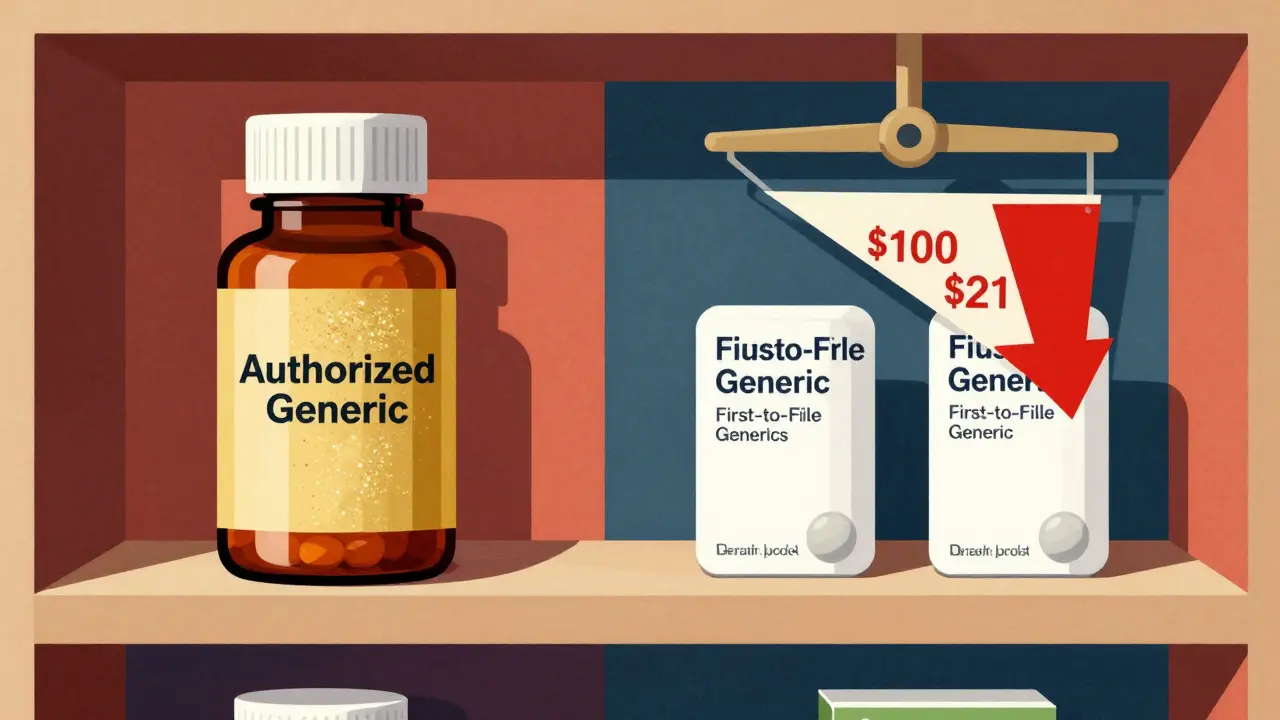
Read moreCost Comparison: Authorized Generics vs First-to-File Generics - What You Really Pay
Authorized generics and first-to-file generics both lower drug costs, but authorized generics drive prices down faster and deeper. Learn how they differ, why prices drop more when both are on the market, and what it means for your prescription costs.
Read moreChronic Pain Conditions: Effective Ways to Manage Lifelong Pain
Chronic pain lasts beyond healing time and affects millions. Learn evidence-based, non-opioid strategies like exercise, CBT, and multidisciplinary rehab that actually improve function and quality of life - backed by CDC, WHO, and Mayo Clinic research.
Read moreHow to Read Prescription Label Directions Like BID, TID, and PRN
Learn how to read common prescription abbreviations like BID, TID, and PRN to take your meds safely and correctly. Understand timing, avoid errors, and know what to ask your pharmacist.
Read more