When you pick up a prescription, you might not think about who made the pill or how much it cost to produce. But behind every bottle of medication - whether it's a brand-name drug or a generic - there's a complex web of labor, regulations, and economics. And the difference in labor costs between generic and brand-name drugs is one of the biggest reasons why generics cost so much less.
Why Generics Cost Less: It’s Not Just the Ingredients
Many people assume generics are cheaper because they use the same active ingredients as brand-name drugs. That’s true - but it’s only part of the story. The real savings come from how those drugs are made. Brand-name drugs are developed from scratch. That means years of research, clinical trials, and regulatory approvals before a single pill hits the shelf. The average cost to bring a new drug to market? Around $2.6 billion. That money doesn’t come from thin air. It comes from pricing the drug high enough to recoup those costs.
Generic manufacturers don’t have that burden. They don’t need to run expensive clinical trials. They don’t need to prove the drug works - because the original brand already did. All they need to prove is that their version is bioequivalent. That cuts out years of work and millions in expenses. But even with that advantage, labor still plays a huge role in how much a generic drug ends up costing.
Labor in Brand-Name Drug Production
For brand-name drugs, labor isn’t just about assembling pills. It’s about managing complexity. From chemists running tests in clean rooms to regulatory specialists documenting every batch, the workforce is deep and specialized. Labor makes up 30% to 40% of total production costs during the early stages. Why? Because brand manufacturers are building systems from the ground up - designing processes, training teams, and meeting strict FDA standards for every step.
Think of it like building a custom house. Every detail matters. A single change in the formulation might require revalidation of the entire production line. That means engineers, quality control staff, and compliance officers all need to be involved. And because brand-name drugs are often produced in smaller batches, labor can’t be spread out over millions of units. The cost per pill stays high.
Labor in Generic Drug Production
Generic production is the opposite. It’s built for scale. Once a generic drug is approved, manufacturers can produce hundreds of millions of pills a year. That massive volume changes everything. Labor costs drop to 15%-25% of total production costs - nearly half the rate of brand-name drugs.
How? Economies of scale. When production volume doubles, generic manufacturers see a 27% drop in unit costs. That’s because they can spread fixed labor expenses - like training, equipment setup, and QA systems - over far more units. A single lab technician can oversee testing for 10,000 batches instead of 100. A packaging line that runs 24/7 doesn’t need more staff to keep up. Efficiency isn’t optional - it’s survival.
But here’s the catch: even with scale, labor in generic manufacturing isn’t cheap because it’s easy. It’s cheap because it’s repetitive and optimized. Quality control alone accounts for over 20% of total production costs. That means inspectors, lab analysts, and documentation specialists are still busy - just doing the same thing over and over, with fewer errors thanks to automation and standardized processes.
The Hidden Labor Costs: Compliance and Quality
Here’s something most people don’t realize: generic drug makers spend nearly as much on compliance as they do on raw ingredients. The FDA requires every batch to be tracked from raw material to finished product. That’s not just paperwork - it’s people. A medium-sized generic company spends about $184,000 a year just to keep its compliance systems running. Add in the cost of filing new drug applications - another $320,000 per drug - and you’re looking at millions in labor-driven overhead.
And it’s not just about paperwork. The "Cost of Quality" model breaks labor expenses into four buckets: prevention (training, planning), appraisal (testing, inspections), internal failure (rework, scrap), and external failure (returns, recalls). For generics, prevention and appraisal make up the biggest slice. That means skilled workers are constantly checking, testing, and verifying - not because they’re being paid more, but because the system demands it.
Geography Matters: Labor Costs Outside the U.S.
Almost 42% of active pharmaceutical ingredients (APIs) for generics are made in India and China. Why? Because labor there costs far less. A worker in a Chinese API plant might earn 70% less than a U.S. technician doing the same job. That doesn’t mean the work is easier. It means the cost structure is different.
But here’s the problem: those lower costs don’t come from better efficiency. They come from lower wages, fewer regulations, and less environmental oversight. According to the U.S. Department of Health and Human Services, these international advantages "artificially depress costs" - meaning U.S. manufacturers can’t compete on price alone. So they respond by outsourcing more. Today, nearly 42% of biosimilar production costs go to contract manufacturers. That turns fixed labor costs into variable ones. When demand drops, you don’t lay off staff - you just reduce your outsourcing.
Competition Drives Labor Efficiency
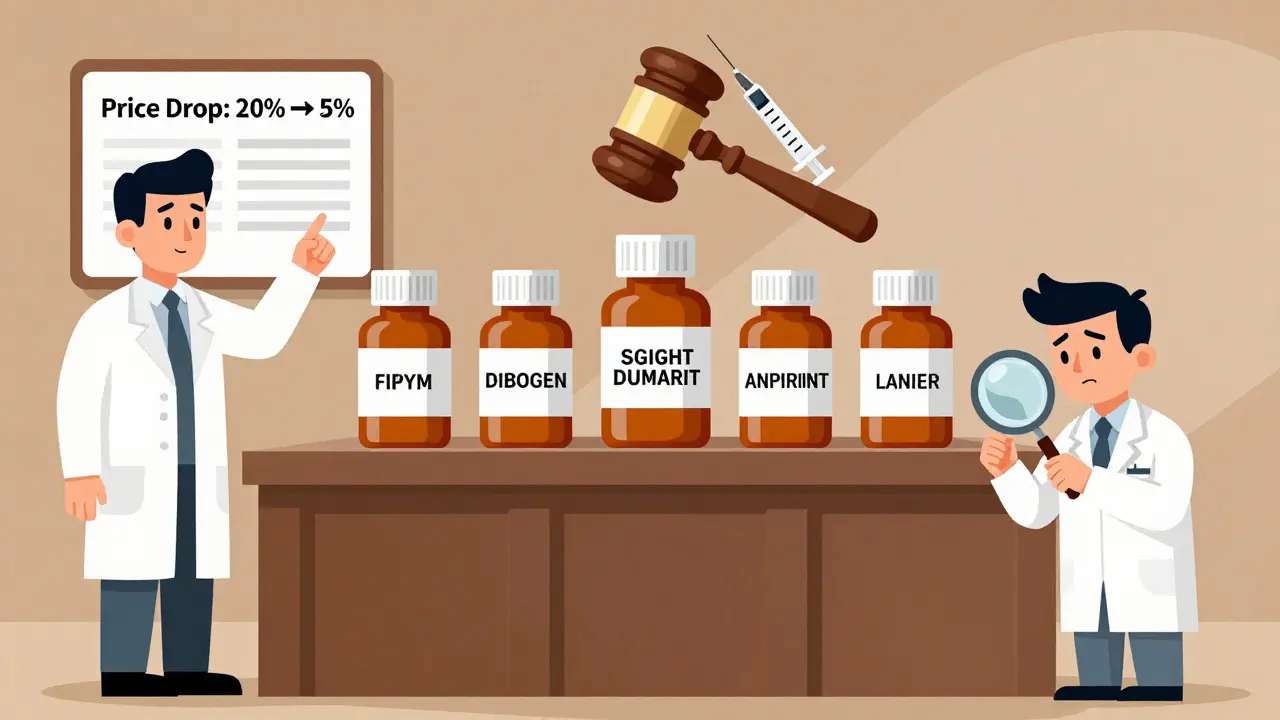
There are over 10,000 generic drugs on the market. And when multiple companies make the same drug, prices crash. The first generic to enter the market might sell at 20% of the brand price. By the time five companies are making it? The price drops to 5% or less.
That kind of pressure forces manufacturers to cut every cost - including labor. Some companies respond by investing in automation. Others cut training hours or hire less experienced staff. The FDA has warned that this could lead to supply shortages and quality issues. A 2023 report noted that "increasing attention" is being paid to whether lower costs are forcing companies to compromise on staffing levels.
But the smartest generic manufacturers are doing the opposite. They’re investing in labor - not cutting it. By training staff to prevent errors, improve testing speed, and reduce rework, they lower their total production cost over time. One manufacturer reduced QC release times by 40% just by improving training. That meant fewer delays, less waste, and higher output - all without hiring more people.
What This Means for You
When you save $50 on a prescription by choosing a generic, you’re not just getting a cheaper version of the same drug. You’re benefiting from a system built on volume, efficiency, and intense competition. The labor behind that bottle isn’t cheaper because it’s unskilled. It’s cheaper because it’s streamlined, standardized, and scaled.
But there’s a flip side. That same system is fragile. If a single API supplier shuts down - or if labor shortages hit overseas factories - supply chains break. And when that happens, shortages hit U.S. pharmacies. The very efficiency that keeps generics affordable also makes them vulnerable.
The next time you fill a generic prescription, remember: you’re not just saving money. You’re part of a global system that balances cost, quality, and scale. And that balance is always being tested - by competition, by regulation, and by the people who make it all happen.

Erin Pinheiro
February 22, 2026 AT 11:22