When you pick up a prescription, you might not realize there are two kinds of generic drugs on the shelf - and they can cost very different amounts. One is made by the original brand company under a different label. The other is made by a rival company that raced to be first to file for approval. These are called authorized generics and first-to-file generics. They’re both technically generic versions of the same brand drug. But their pricing, timing, and market impact are worlds apart.
What Exactly Is an Authorized Generic?
An authorized generic is the exact same pill, capsule, or injection as the brand-name drug - same active ingredient, same factory, same packaging - just without the brand name on it. It’s made by the original manufacturer, often under a licensing deal with a generic company. You won’t find it advertised on TV. You’ll see it labeled with the generic name and maybe a small logo from the brand company.
For example, if you take the brand-name drug Lipitor (atorvastatin), the authorized generic is just “atorvastatin” sold by Pfizer or one of its partners. It’s chemically identical. No testing needed. No delays. It hits the market as soon as the brand decides to launch it - sometimes even before the first generic enters.
What Is a First-to-File Generic?
The first-to-file generic is the underdog. It’s made by a different company that spent years and millions of dollars to prove its version works just like the brand. They file an Abbreviated New Drug Application (ANDA) with the FDA. If they’re the first to do so and challenge a patent successfully, they get 180 days of exclusive rights to sell their version - no competition allowed.
This exclusivity is huge. During those 180 days, the first-filer can charge more than other generics. They’re the only game in town. That’s why companies fight tooth and nail to be first. The payoff can be hundreds of millions of dollars. But here’s the twist: that exclusivity doesn’t block authorized generics. And when an authorized generic shows up during that window, everything changes.
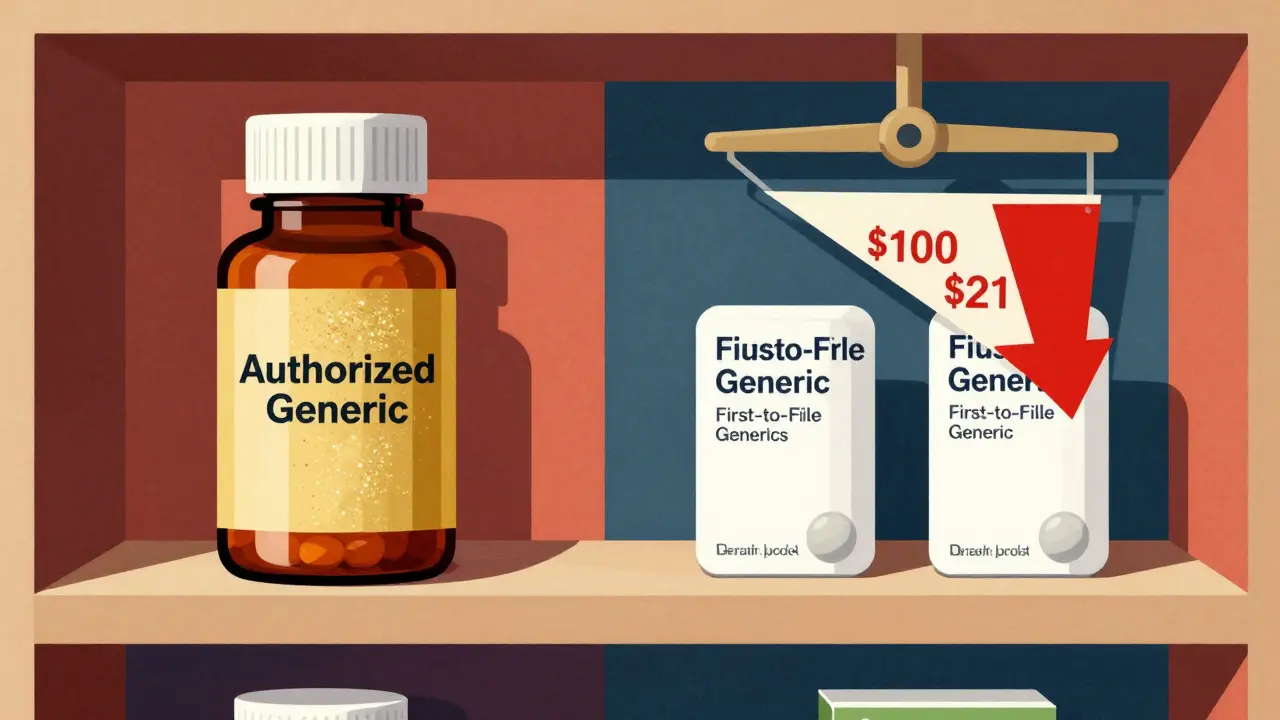
Price Differences: The Real Numbers
Let’s talk money. In 2011, the Federal Trade Commission (FTC) looked at 95 drugs and found that when only the first-to-file generic was on the market, the average retail price was about 14% lower than the brand-name version. Sounds good, right? But when an authorized generic entered the same market during those 180 days, the price dropped even further - to 18% below the brand. That’s a 4-point swing just from adding one more player.
For pharmacies buying the drugs wholesale, the difference is even sharper. Without an authorized generic, they paid about 20% less than the brand. With an authorized generic competing, that jumped to 27% less. That’s a 7-point increase in discount - meaning pharmacies make more profit per prescription.
By 2019, the FDA confirmed this trend across hundreds of drugs. When only one generic was available, the average manufacturer price was 39% below the brand. Add one authorized generic? That jumped to 54% below. Add four generics total? Prices dropped to 79% less than the brand. Six or more? Over 95% cheaper.
So yes - more competition means lower prices. But the real surprise? The first authorized generic often triggers the biggest price drop of all.

Why Authorized Generics Hit Harder
Think of the first-to-file generic as the only car on a highway. It can set its own speed. Now imagine another car - identical, from the same manufacturer - pulls onto the same road. Suddenly, the first car has to slow down. It can’t charge premium prices anymore. That’s what happens with authorized generics.
The first-filer didn’t expect this. They thought they had the market to themselves. But the brand company - the one who originally made the drug - decides to sell their own version under a generic label. Why? Because they’re trying to capture some of the profit before other generics flood in. It’s a strategic move. And it works.
The FTC found that when an authorized generic enters during the 180-day exclusivity window, the first-filer’s revenue drops by 40% to 52%. And that hit doesn’t fade. Even 30 months later, their sales are still significantly lower than they would’ve been without the authorized generic.
Who Wins and Who Loses?
Consumers win. Lower prices mean lower out-of-pocket costs and lower insurance claims. The healthcare system saves money. Pharmacies win too - higher margins per prescription.
The brand company wins. They still make money off their own authorized generic. They don’t lose the whole market to a rival. They control the timing and the supply.
The first-to-file generic loses. Their golden 180 days get cut short. Their profits shrink. Some companies even avoid filing for patents if they know a brand will launch an authorized generic. But here’s the twist: the FTC found no evidence that this reduces the number of patent challenges overall. Generic companies still file. They just know the reward might be smaller.
Some critics worry that authorized generics could discourage innovation. If a generic company knows a brand might undercut them, will they still risk millions to challenge a patent? The data says yes - they still do. The 180-day window is still worth it, even with competition.
What About Long-Term Prices?
After the 180 days, more generics flood in. That’s when prices really crash. But authorized generics don’t vanish. Many stick around - sometimes for years. They’re often priced lower than other generics because they come from the original manufacturer. They’re reliable. Pharmacies trust them.
Research from Health Affairs in 2023 showed that about 20% of authorized generics disappeared after five years - meaning they weren’t sold in Medicare data. But the ones that stuck? They kept prices low. They didn’t become expensive niche products. They stayed competitive.

How This Affects You at the Pharmacy
If you’re paying cash for a generic drug, always ask: “Is this an authorized generic?” You might get the same drug for less. Some pharmacies stock both. The authorized generic might be on the same shelf, just labeled differently.
If you’re on insurance, your plan might favor one over the other. But if you have a choice, go for the lowest price. It doesn’t matter if it’s branded or not - the active ingredient is the same.
And if your prescription switches from one generic to another, don’t panic. That’s normal. It doesn’t mean the drug changed. It just means the pharmacy switched suppliers - maybe to save money.
What’s Changing in the Market?
The FDA’s Generic Drug User Fee Amendments (GDUFA), updated in 2022, have sped up approval times. First-time approvals now happen in about 66% of cases - up from 20% a decade ago. That means more generics enter faster. That’s good for competition.
But brand companies are getting smarter. They’re launching authorized generics earlier. Sometimes, they even settle patent lawsuits by agreeing to launch an authorized generic - a tactic the FTC is watching closely. In 2013, the Supreme Court ruled that these kinds of deals can be anti-competitive if they’re meant to delay other generics.
And don’t forget: brand companies sometimes release new versions - like extended-release pills - right after a generic hits. That can shrink the market for first-to-file generics by up to 29%. It’s a legal way to keep customers loyal.
Final Takeaway
Authorized generics aren’t a loophole. They’re a tool. And they’re one of the most effective ways to drive down drug prices fast. They don’t replace first-to-file generics - they force them to compete. And that’s good for you.
The bottom line? More competition = lower prices. Whether it’s a first-to-file generic or an authorized one, the more options you have, the better off you are. Don’t assume all generics are the same. Ask questions. Compare prices. And remember - the cheapest option isn’t always the one with the familiar brand name on it.
Are authorized generics the same as brand-name drugs?
Yes. Authorized generics are identical to the brand-name drug in active ingredient, dosage, strength, and how they work. They’re made in the same factory, often on the same production line. The only difference is the label - no brand name, no marketing. You get the exact same drug, just cheaper.
Why are authorized generics cheaper than the brand but sometimes more expensive than other generics?
They’re usually cheaper than the brand because there’s no marketing cost. But during the 180-day exclusivity window, they can be priced higher than other generics because they’re the only competition to the first-filer. Once more generics enter, prices drop across the board. Authorized generics often stay low because they come from the original manufacturer and don’t need to recoup R&D costs.
Can I ask my pharmacist for an authorized generic?
Absolutely. Pharmacists often carry multiple generic versions. Ask if they have the authorized generic - it might be priced lower than other generics, especially during the first 180 days after brand entry. Some insurance plans even prefer it because it’s reliable and cost-effective.
Do authorized generics delay other generics from entering the market?
No. The FTC found no evidence that authorized generics reduce the number of patent challenges or delay other generics. Generic companies still file ANDAs and fight patents. The 180-day exclusivity remains valuable, even with an authorized generic competing. In fact, the presence of an authorized generic often signals that the brand’s patent is weak - which can encourage more generic entries later.
Why do brand companies make authorized generics?
It’s a business move. Instead of losing all sales to a rival generic, they keep some of the profit by selling their own version under a generic label. It’s a way to soften the financial blow of generic competition. It also lets them control quality and supply. And if they launch it as part of a patent settlement, it can help avoid costly lawsuits.
Is it safe to switch between authorized generics and other generics?
Yes. All generics - whether authorized or not - must meet FDA standards for bioequivalence. That means they work the same way in your body. Switching between them is safe and common. If you notice a change in how you feel, talk to your doctor - but it’s rarely because of the generic version. More often, it’s due to inactive ingredients, which vary slightly between manufacturers.

Hilary Miller
January 21, 2026 AT 23:10Just asked my pharmacist for the authorized generic yesterday - saved me $12 on my cholesterol med. Same pill, no brand hype. Why pay more?
Neil Ellis
January 23, 2026 AT 13:10This is one of those hidden gems in healthcare that nobody talks about - like finding out your favorite coffee shop uses the exact same beans as the fancy boutique, but charges half. Authorized generics are the quiet heroes of the pharmacy shelf. They don’t need a billboard or a jingle. Just a label change and a lower price. And somehow, that’s enough to shake the whole system. It’s beautiful, really - capitalism with a conscience.
Lana Kabulova
January 24, 2026 AT 15:43Wait - so if the brand company makes the authorized generic, doesn’t that mean they’re still profiting off the same drug? And they’re the ones who patented it in the first place? That feels like a loophole disguised as competition. Also, why do we even call it ‘generic’ if it’s the same company? Just say it’s a ‘rebranded brand’ - that’s what it is.
Daphne Mallari - Tolentino
January 26, 2026 AT 03:33It is, of course, entirely unsurprising that the pharmaceutical industry has engineered a system where the very entity that monopolized the drug for two decades now monetizes its own obsolescence under a different label. The irony is not lost on those of us who have watched the cost of insulin rise 500% over the past decade - while authorized generics, quietly, undercut the market by 79%. A system that rewards legal maneuvering over patient access is not a system - it is a performance.
Keith Helm
January 27, 2026 AT 20:33Per FDA guidelines, all generics must demonstrate bioequivalence within a 90% confidence interval of 80-125%. Authorized generics are not subject to this testing - they are identical by definition. Therefore, their inclusion in the market is not ‘competition’ - it is the original product being reintroduced under a different nomenclature. The FTC’s interpretation is misleading.
Alec Amiri
January 29, 2026 AT 10:01First-to-file generics are the real villains here. They sit on their 180-day monopoly like a dragon on gold, charging $200 for a pill that costs $2 to make. Then when the brand drops an authorized generic? They scream ‘unfair!’ Nah. You got your shot. Now get out of the way. People are dying because you’re greedy.
Lauren Wall
January 31, 2026 AT 07:14I don’t care what label it’s under. If it’s the same pill, why does anyone still buy the brand? You’re just funding corporate PR.
Sarvesh CK
February 1, 2026 AT 19:45It is worth noting that the structural dynamics described herein reflect not merely a market phenomenon, but a profound recalibration of incentive structures within pharmaceutical innovation. The authorized generic, in its essence, functions as a market equilibrium mechanism - a form of self-regulation by the incumbent, wherein the threat of preemptive commoditization serves to moderate the monopolistic rent-seeking behavior of first-filers. One might argue that this represents a form of corporate pragmatism, albeit one that remains deeply embedded within a profit-driven framework. Nevertheless, the net effect - a measurable reduction in out-of-pocket expenditures for patients - cannot be dismissed as trivial. The ethical imperative, then, lies not in condemning the mechanism, but in ensuring its transparency and accessibility to all segments of the population, regardless of insurance status or socioeconomic standing.
arun mehta
February 3, 2026 AT 03:36Love this breakdown! 🙌 Seriously, most people don’t even know this exists. I always ask for the authorized generic now - my mom’s blood pressure med dropped from $45 to $8. Same pill. Same factory. Just no fancy logo. 🇮🇳🇺🇸 #PharmaTransparency
Oren Prettyman
February 5, 2026 AT 01:30Let’s be honest - this entire discussion is a distraction. The real issue isn’t whether authorized generics are ‘better’ or ‘fairer’ - it’s that we live in a system where a single pill can cost $100, $20, or $5 depending on which corporation controls the label. The fact that we’re debating which version of exploitation is less offensive is itself a symptom of a broken system. Why are we not demanding price caps? Why are we not nationalizing drug manufacturing? Why are we still pretending that corporate self-interest, even when slightly moderated, is a valid substitute for public health policy? This isn’t a nuanced market analysis - it’s a plea for us to stop pretending that capitalism can fix what it broke.