LDAA Dose Calculator
How This Calculator Works
This tool calculates the safe reduced dose of azathioprine needed when combined with allopurinol. Never take full-dose azathioprine with allopurinol - it's dangerous.
The standard LDAA protocol requires:
- Azathioprine dose reduced to 25-33% of original amount
- Allopurinol fixed at 100 mg daily
- TPMT testing before starting
Your Safe LDAA Dose
Important Safety Information
Never take full-dose azathioprine with allopurinol. This can cause severe bone marrow suppression and life-threatening neutropenia.
Always get TPMT testing before starting. Patients with low TPMT activity should not use this combination.
Your doctor will monitor your blood counts and metabolite levels regularly. Missing monitoring can lead to serious complications.
When you’re on azathioprine for Crohn’s disease, ulcerative colitis, or autoimmune hepatitis, you’re counting on it to calm your immune system. But for some people, the drug doesn’t work - or worse, it starts damaging the liver. That’s where allopurinol comes in. Not as a gout drug this time, but as a metabolic switch that redirects how azathioprine is processed in your body. Together, they form a powerful, underused combo called LDAA - low-dose azathioprine with allopurinol. And for the right patients, it’s life-changing.
Why Azathioprine Can Backfire
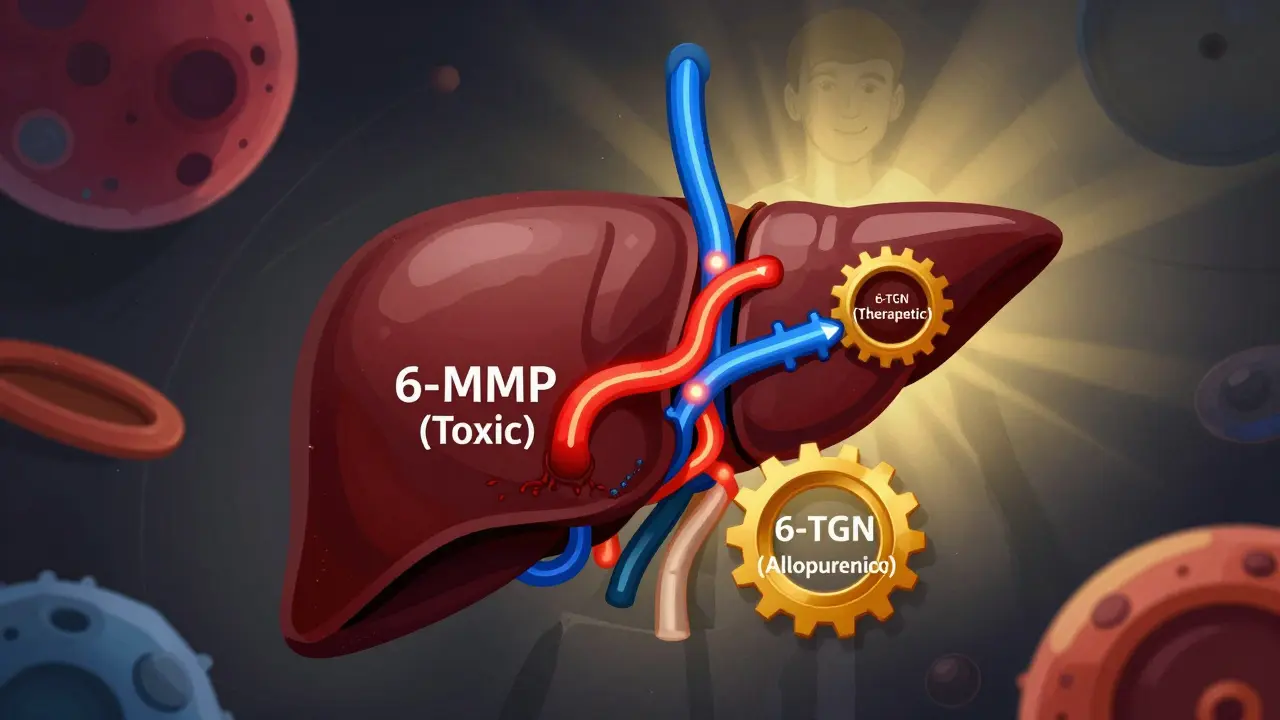
Azathioprine breaks down into 6-mercaptopurine (6-MP), which then splits into three different paths inside your cells. One path makes 6-thioguanine nucleotides (6-TGN), the good stuff that suppresses inflammation. Another path makes 6-methylmercaptopurine (6-MMP), a toxic byproduct that fries your liver. And the third just flushes it out as useless waste. The problem? About 15-20% of people have high levels of an enzyme called TPMT. That means their bodies are wired to make way too much 6-MMP. These are called ‘hypermethylators.’ They get liver damage, not relief. Their 6-TGN levels stay low. The drug isn’t working - and they’re getting sicker from the side effects.How Allopurinol Changes the Game
Allopurinol was designed to treat gout. It blocks xanthine oxidase, an enzyme that breaks down purines. But in this case, that same blockage is a gift. By shutting down one of the dead-end pathways for 6-MP, allopurinol forces more of the drug down the 6-TGN route. Think of it like rerouting traffic away from a jammed highway. The result? A 70-90% drop in 6-MMP. A 2- to 5-fold jump in 6-TGN. Liver enzymes normalize. Inflammation drops. Patients who couldn’t tolerate azathioprine before suddenly go into remission. But here’s the catch: you can’t just add allopurinol to a full dose of azathioprine. That’s how people end up in the hospital with zero white blood cells. You have to slash the azathioprine dose - to 25-33% of what you’d normally use. That means if you were on 150 mg, you drop to 50 mg. Then you add 100 mg of allopurinol daily.Who Benefits Most?
This combo isn’t for everyone. It’s targeted. You’re a good candidate if:- Your liver enzymes are high on standard azathioprine
- Your 6-MMP levels are above 5,700 pmol/8×10⁸ RBCs
- Your 6-TGN levels are below 230 pmol/8×10⁸ RBCs
- You’ve tried other drugs like anti-TNFs and they failed or weren’t tolerated
- You have normal or high TPMT activity (above 14.2 U/mL)

The Monitoring Protocol That Saves Lives
This isn’t a set-it-and-forget-it treatment. You need strict, frequent monitoring. Here’s what works:- Start azathioprine at 50 mg/day and allopurinol at 100 mg/day
- Get a complete blood count (CBC) every week for the first four weeks
- Check 6-TGN and 6-MMP levels at 4 weeks
- Target 6-TGN between 230 and 450 pmol/8×10⁸ RBCs
- Keep 6-MMP under 2,800 pmol/8×10⁸ RBCs
- After month one, switch to CBC every two weeks for three months, then monthly
Real Results, Real Risks
Studies show this combo works. In one trial, 70% of hypermethylator IBD patients went into remission with LDAA. Only 35% did on standard azathioprine. Liver enzymes returned to normal in 85-90% of cases. But the risks are real. Before dose reduction protocols were standardized, leukopenia rates hit 40%. Now, with proper dosing and monitoring, it’s under 10%. That’s a huge difference. In Australia, Europe, and Canada, LDAA is now a standard second-line option for IBD. In the U.S., adoption is slower. Some doctors still remember the 1981 FDA warning about fatal bone marrow suppression - and they avoid the combo entirely. But those warnings were for unadjusted doses. Modern protocols are safe.
Cost vs. Biologics: A Clear Advantage
Azathioprine costs about $50 a year. Allopurinol is $20. Together, LDAA runs $1,200-$1,800 annually. Compare that to Humira or Remicade - $30,000 to $50,000 per year. For patients without good insurance, or in countries with limited healthcare access, LDAA is a game-changer. It’s not just cheaper. It’s effective. A 2023 study in Hepatology showed 82% of autoimmune hepatitis patients responded to LDAA after failing standard therapy. That’s not a backup plan - that’s a first-line option for the right patient.What’s Next?
The future of LDAA is precision. Right now, you need a blood test to measure 6-TGN and 6-MMP. That takes days, costs money, and isn’t available everywhere. But two companies are developing point-of-care tests that can give results in under an hour. Phase 3 trials are underway. If they succeed, LDAA could become as routine as checking blood pressure. The European Crohn’s and Colitis Organisation (ECCO) and the American Gastroenterological Association (AGA) now both recommend LDAA for azathioprine-intolerant patients. It’s no longer experimental. It’s evidence-based.Final Thoughts
LDAA isn’t magic. It’s math. It’s metabolism. It’s knowing which enzyme is overactive and how to gently turn it down. It’s not for every patient. But for the 1 in 5 with high TPMT and liver damage on azathioprine? It’s the difference between continuing to suffer and finally getting control. If you’re on azathioprine and your liver enzymes are up, or your symptoms aren’t improving, ask your doctor about metabolite testing. Don’t assume the drug isn’t working. Maybe it’s just going the wrong way - and a simple switch could fix it.Can I take azathioprine and allopurinol together without reducing the azathioprine dose?
No. Taking full-dose azathioprine with allopurinol is extremely dangerous. It can cause severe, life-threatening bone marrow suppression. The azathioprine dose must be reduced to 25-33% of the original amount - typically 50 mg/day - before adding allopurinol. Never combine them without strict medical supervision and dose adjustment.
How long does it take for LDAA to start working?
Most patients see improvement in liver enzymes within 4-8 weeks. Clinical remission - reduced diarrhea, less bleeding, fewer flares - usually takes 3 to 6 months. Blood levels of 6-TGN peak around 4 weeks, which is why testing at that point is critical to confirm the right dose.
Is LDAA safe for patients with kidney problems?
No. LDAA is contraindicated in patients with severe renal impairment (creatinine clearance below 30 mL/min). Allopurinol and its active metabolite, oxypurinol, are cleared by the kidneys. If your kidneys aren’t working well, these drugs can build up and increase the risk of toxicity. Alternative treatments should be used instead.
Do I still need to monitor my blood if I feel fine on LDAA?
Yes. You can feel perfectly fine and still develop low white blood cell counts. Neutropenia often has no symptoms until it’s severe. Weekly CBCs for the first month, then every two weeks for three months, then monthly, are essential. Skipping monitoring is the leading cause of serious complications.
Can LDAA be used for conditions other than IBD?
Yes. LDAA is increasingly used in autoimmune hepatitis, lupus nephritis, and some forms of vasculitis. A 2023 study in Hepatology showed 82% of autoimmune hepatitis patients responded to LDAA after failing standard azathioprine therapy. The same metabolic logic applies - redirecting toxic metabolites to improve safety and efficacy.
What if my 6-TGN levels are too high on LDAA?
If your 6-TGN exceeds 450 pmol/8×10⁸ RBCs, you’re at increased risk for myelosuppression. Your doctor will reduce your azathioprine dose further - possibly to 25 mg/day or lower - and may temporarily pause allopurinol. Most patients can be safely re-titrated with close monitoring. Never adjust the dose yourself.
Is TPMT testing required before starting LDAA?
Yes. TPMT testing is essential. Patients with low or absent TPMT activity (below 5 U/mL) should not receive any thiopurine, including LDAA, due to high risk of severe bone marrow suppression. TPMT testing helps identify who will benefit and who is at risk. It’s a standard of care, not optional.

Crystal August
January 19, 2026 AT 01:14This is why medicine is broken. You're telling me we're using a 1960s drug combo as a 'life-changing' solution when we have biologics that actually work? It's just band-aiding a system that should've been fixed decades ago.
Nadia Watson
January 19, 2026 AT 03:02I've been on azathioprine for 7 years with UC, and my liver enzymes spiked last year. My GI doc mentioned LDAA but said we'd need to wait for metabolite testing. It took 3 months to get the blood work done. When we finally did, my 6-MMP was over 7,000. We switched to the low-dose combo, and within 6 weeks, my ALT dropped from 142 to 38. I still get weekly blood draws, but I haven't had a flare since. It's not magic-it's science. Just wish more doctors knew about it.
Shane McGriff
January 19, 2026 AT 12:25Let me just say this: if your doctor hasn't mentioned metabolite testing, they're not keeping up. I had a patient last month who was on 150mg azathioprine for 18 months with zero improvement and elevated LFTs. We checked her 6-TGN and 6-MMP-classic hypermethylator. Cut the azathioprine to 45mg, added 100mg allopurinol, and within 8 weeks, she was in remission. No biologics. No infusion center. Just smart pharmacokinetics. This isn't fringe medicine-it's the standard of care in Europe. Why are we still debating it here?
Jacob Cathro
January 19, 2026 AT 13:40so like... allopurinol is just a 'traffic rerouter'? lol. i mean, if you think about it, isn't this just the pharmaceutical industry's way of making old drugs look new again? like, they got 2 generic pills and slapped a fancy acronym on it. LDAA? sounds like a bad startup name. also, why does everyone ignore that this combo can still wipe out your WBCs? i know a guy who went from 'feeling fine' to sepsis in 4 days. no one warned him. #pharmaconspiracy
Paul Barnes
January 19, 2026 AT 15:33The assertion that LDAA is 'underused' is misleading. It is not underused-it is underprescribed due to legitimate safety concerns, and the data supporting its efficacy is largely retrospective. Prospective, randomized trials remain limited. The 70% remission rate cited is from small, single-center studies. Until larger, multi-center trials confirm these outcomes, caution is warranted.
pragya mishra
January 21, 2026 AT 11:04Why are you all so obsessed with American medical protocols? In India, we've been using this combo for 15 years. My uncle had autoimmune hepatitis-he couldn't afford biologics. LDAA saved his life. Now you people act like it's some new discovery. It's not. It's just that your healthcare system is too expensive to allow common sense.
Andy Thompson
January 22, 2026 AT 03:31ALLOPURINOL IS A GOUT DRUG. THEY'RE USING IT TO CONTROL YOUR IMMUNE SYSTEM? WHO TOLD THEM TO DO THAT? I bet this is all part of the WHO's plan to control the population through drug manipulation. First they make you sick, then they sell you a 'combo' that costs $1,200 a year while biologics cost $50k. That's not medicine-that's a tax. And don't tell me about 'TPMT testing.' They don't test you for anything. They just take your blood and disappear. #DeepStatePharma
sagar sanadi
January 22, 2026 AT 17:03so you're telling me the solution to a drug that doesn't work is... to add another drug? genius. why not just add aspirin and call it LDAA+? also, 82% response rate? that's the same number they used for the placebo pills in the 90s. i'm not buying it.
kumar kc
January 23, 2026 AT 23:09If you need a combo to make azathioprine work, you shouldn't be on it in the first place. Stop trying to fix broken systems. Just switch to biologics.
Thomas Varner
January 25, 2026 AT 04:31I’ve been on LDAA for 14 months now. My 6-TGN is at 312, 6-MMP at 1,900. I feel great. But I still check my CBC every week, even though I feel fine. I know people who skipped monitoring and ended up in the ER. I’m not taking chances. Also, the cost difference? My biologic co-pay was $1,200/month. This? $30/month. I don’t care what the FDA says-I’m keeping this regimen.
Art Gar
January 25, 2026 AT 20:34While the pharmacokinetic rationale for LDAA is sound, the clinical adoption remains inconsistent across jurisdictions. The absence of standardized dosing algorithms and the variability in metabolite assay methodologies contribute to suboptimal implementation. Until national guidelines explicitly endorse LDAA as a first-line alternative in hypermethylators, its use should remain confined to specialized centers with access to therapeutic drug monitoring.
Edith Brederode
January 27, 2026 AT 04:45Thank you for writing this. 💙 I was terrified when my doctor suggested LDAA, but after reading this, I feel so much more confident. My liver enzymes were through the roof, and I was ready to give up. Now I’m on it for 3 months and my flares are down 80%. I still get nervous about blood draws, but I know they’re saving my life. You’re right-it’s math, not magic. And I’m so glad I didn’t listen to the fear.
clifford hoang
January 28, 2026 AT 01:07Think about it: azathioprine was developed in the Cold War as a potential immunosuppressant for soldiers. Allopurinol? Originally a byproduct of nuclear research. Now they're combining them? This isn't medicine-it's a chemical experiment on human beings. Who funded these studies? Who owns the patents? And why is no one asking why we're still using 1950s chemistry to treat 21st-century autoimmune diseases? The answer? Because the system doesn't want you to heal. It wants you to stay sick, on meds, for life.