When you pick up a generic pill from the pharmacy, you’re not getting a cheaper copy-you’re getting a medicine that has passed one of the strictest tests in modern medicine: bioequivalence. This isn’t just a buzzword. It’s the legal and scientific guarantee that the generic version of your drug behaves in your body exactly like the brand-name version. The key? How fast and how much of the active ingredient gets into your bloodstream. And that’s where absorption rates come in.
What Bioequivalence Really Means
Many people think generics are just ‘close enough.’ That’s wrong. The U.S. Food and Drug Administration (FDA) doesn’t allow ‘close enough.’ It demands exact matches-within a very narrow window. For a generic drug to be approved, its absorption rate must be virtually identical to the brand-name drug. Not similar. Not nearly the same. Identical.
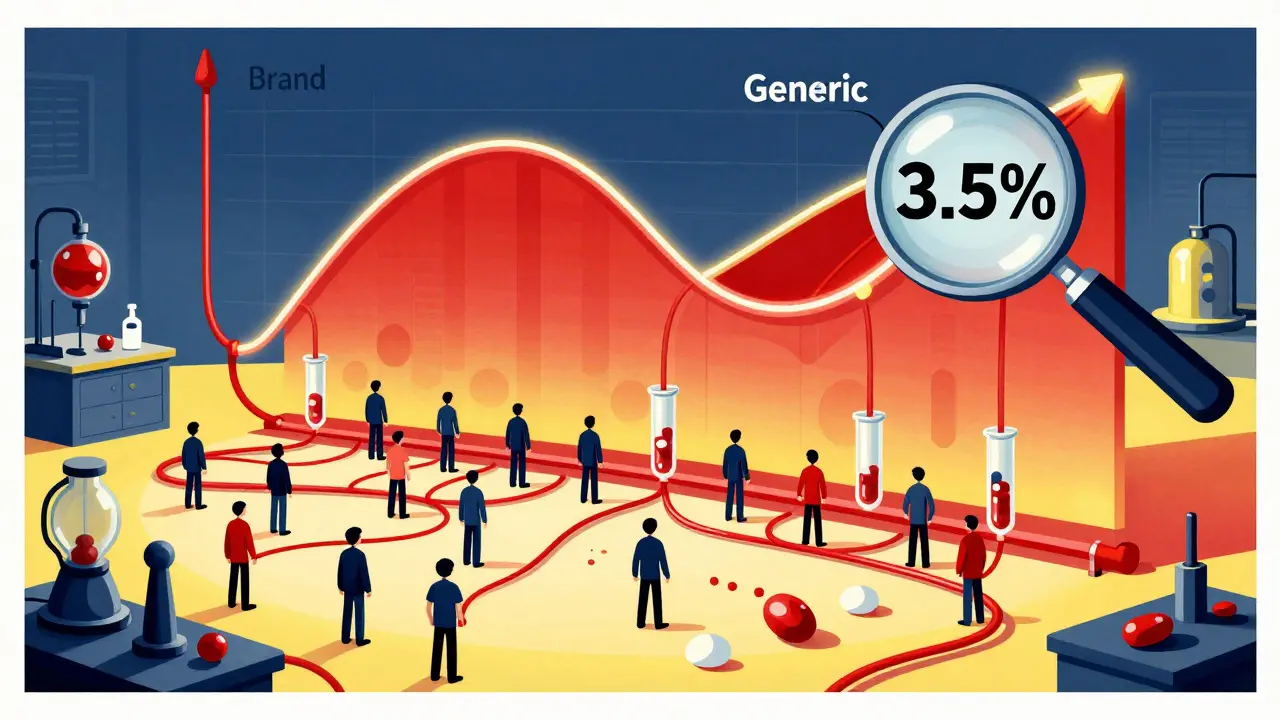
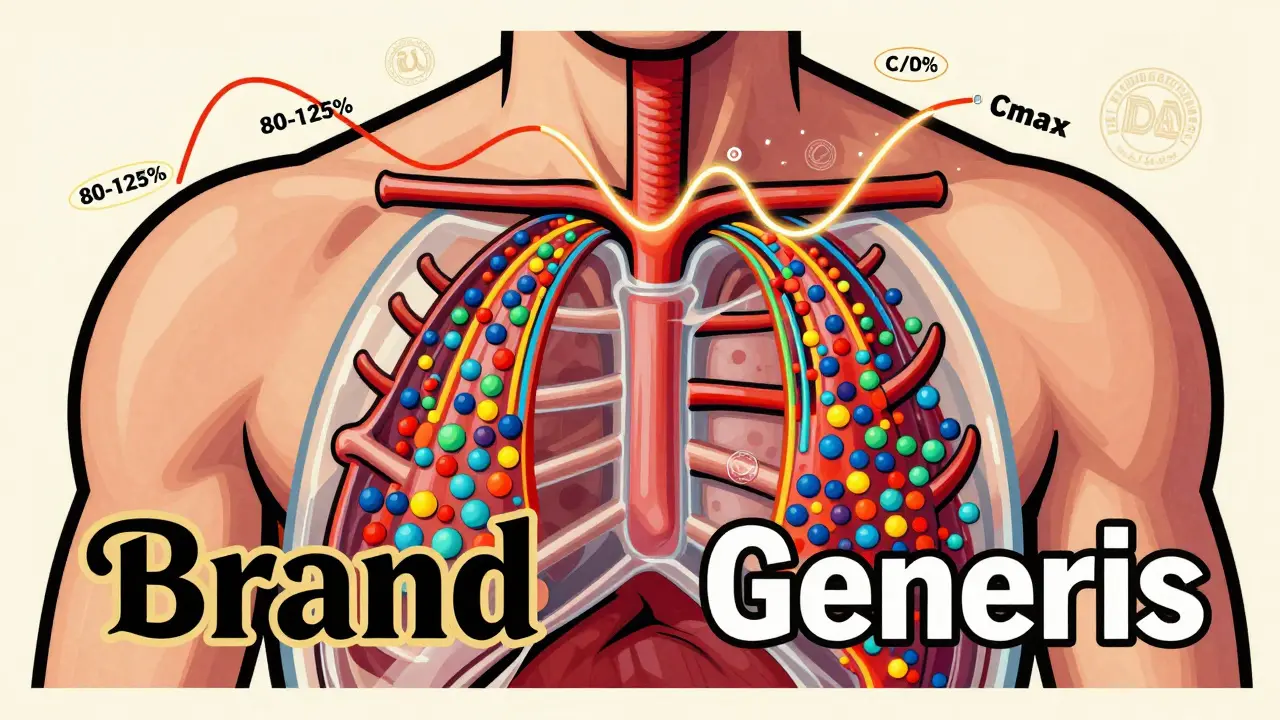
The rule is simple: the amount of drug your body absorbs from the generic version must fall between 80% and 125% of what you get from the brand. That’s not a 45% variation, as some rumors claim. It’s a statistical range that ensures the average difference is less than 4%. In fact, studies of over 2,000 generic drugs show the average difference in absorption is just 3.5%. That’s less than the natural variation your body has when you take the same pill twice on different days.
This isn’t guesswork. It’s measured using two precise metrics: AUC (Area Under the Curve) and Cmax (Maximum Concentration). AUC tells you how much of the drug gets into your system over time-the total exposure. Cmax tells you how quickly it gets there-the speed of absorption. Both must land within the 80-125% range when compared to the brand-name drug. And the FDA doesn’t just look at averages. The entire 90% confidence interval for both measurements must fit inside that window. If even one point outside it slips through, the drug gets rejected.
How the Testing Works
Before a generic drug hits the shelf, it goes through what’s called a crossover bioequivalence study. About 24 to 36 healthy volunteers take both the brand-name drug and the generic version, each in a different order, with a clean break between doses. Blood samples are taken over several hours to map out how the drug moves through the body. These curves are then analyzed using advanced statistical models.
It’s not enough to say, ‘It works fine.’ The data must prove it. The FDA requires this for every single generic drug approved in the U.S. The same standard applies in Europe through the European Medicines Agency. Japan is even stricter for some drugs, requiring an 85-115% range. But the U.S. and EU standards are globally recognized as the gold standard.
Even more impressive? The testing doesn’t stop after approval. The FDA keeps watching. Through its Adverse Event Reporting System, they track real-world outcomes. Between 2008 and 2023, out of more than 14,000 approved generics, only 12 cases raised possible concerns about therapeutic equivalence. That’s a failure rate of 0.08%.
Dissolution Isn’t the Same as Absorption
You might hear complaints that a generic pill dissolves slower than the brand. That’s true in some cases. A 2014 study found that more than half of the generic drugs tested had different dissolution rates in lab conditions-some slower, some faster. Generic nifedipine dissolved much slower. Generic amoxicillin dissolved faster. That sounds alarming, right?
But here’s the catch: dissolution in a test tube doesn’t equal absorption in your body. A pill might dissolve slowly in water but still release its drug quickly in your stomach. Or it might dissolve fast but get trapped in your gut before absorption. The body isn’t a petri dish. What matters is what ends up in your blood-and that’s what the bioequivalence study measures.
The FDA knows this. That’s why they don’t reject generics based on dissolution alone. As long as the blood levels match, the pill’s shape, color, or how fast it breaks down in water doesn’t matter. The only thing that counts is the result inside you.
Why Some Patients Feel Different
Despite the data, people still report feeling different on generics. Online forums like Reddit and Inspire are full of stories: ‘My thyroid meds don’t work anymore.’ ‘I’m more anxious on the generic antidepressant.’
These aren’t imaginary. But they’re rare-and usually not about absorption. A 2023 meta-analysis of 47 studies involving nearly 10,000 patients found no difference in outcomes between generic and brand-name cardiovascular drugs. The same goes for most other drug classes.
So why the complaints? Three big reasons:
- Placebo effect: If you believe generics are inferior, your brain can make you feel worse-even if the drug is identical.
- Switching from a stable brand: If you’ve been on the same brand for years, your body is used to it. Even a 1% shift in absorption can feel noticeable, especially for drugs like levothyroxine or bupropion, where small changes affect mood or energy.
- Manufacturing differences: While absorption is tightly controlled, inactive ingredients (fillers, dyes, coatings) can vary. Some people are sensitive to those, leading to stomach upset or rashes-not reduced effectiveness.
For drugs with a narrow therapeutic index-like warfarin, digoxin, or phenytoin-the rules are even tighter. The FDA requires absorption to stay within 90-111% for these. That’s because a tiny change in blood level can mean the difference between a clot and a bleed, or a seizure and calm.
What the Orange Book Tells You
The FDA’s Orange Book is the official list of all approved drugs and their therapeutic equivalence ratings. It’s not a secret document. It’s public. And it’s your best tool.
Drugs marked with an ‘A’ rating are considered therapeutically equivalent to the brand. That means you can switch confidently. Drugs with a ‘B’ rating? Those are flagged because they have potential bioequivalence issues-either from past problems or because they’re complex (like inhalers or topical creams). If your doctor prescribes a ‘B’ drug, they should explain why.
Most generics are ‘A’ rated. In fact, 49 U.S. states allow pharmacists to automatically substitute an ‘A’ rated generic unless the doctor says ‘dispense as written.’ That’s how confident the system is.

Why This Matters for You
Generics make up 90% of all prescriptions filled in the U.S. But they cost only 23% of what brand drugs do. That’s how we keep healthcare affordable. Without strict bioequivalence rules, we’d be trading savings for risk. But the system works.
Here’s what you should do:
- If you’re switching from brand to generic, give it a few weeks. Your body needs time to adjust.
- If you feel worse, don’t assume it’s the drug. Talk to your doctor or pharmacist. Check if it’s a ‘B’ rated product.
- For narrow therapeutic index drugs, ask if your pharmacy always uses the same generic manufacturer. Consistency helps.
- Don’t avoid generics because of myths. The science is clear: they work.
And if you’re still unsure? Look up your drug in the FDA’s Orange Book. See its rating. Ask your pharmacist for the manufacturer name. Knowledge removes fear.
The Future of Generic Drugs
The FDA is now using computer modeling to predict absorption without always needing human studies. This will speed up approvals for simple generics and free up resources for complex ones-like biosimilars and inhalers. But the core rule won’t change: if it doesn’t match the brand in your bloodstream, it won’t get approved.
By 2027, the FDA aims to review 90% of generic applications in 10 months or less. That’s faster, not looser. The standards are staying firm because the evidence is too strong to ignore.
Generics aren’t a compromise. They’re a triumph of science, regulation, and public health. They let millions of people afford their medicine without sacrificing safety. And that’s why the absorption rate isn’t just a number-it’s a promise.
Are generic drugs really as effective as brand-name drugs?
Yes. Every generic drug approved by the FDA must prove it delivers the same amount of active ingredient into your bloodstream at the same rate as the brand-name version. Studies show the average difference in absorption is just 3.5%, far smaller than natural body variation. The FDA’s own data confirms therapeutic equivalence for over 99.9% of approved generics.
Why do some people say generics don’t work for them?
Some people notice changes when switching due to placebo effects, sensitivity to inactive ingredients (like dyes or fillers), or because they were stable on a specific brand for years. For drugs like levothyroxine or bupropion, even small shifts can feel noticeable. But clinical studies show no real difference in outcomes. If you feel worse, talk to your doctor-don’t assume the drug failed.
Can generic drugs have different side effects?
The active ingredient is identical, so the core side effects are the same. But inactive ingredients can differ between brands and generics. Some people react to dyes, lactose, or coatings in one version but not another. These aren’t drug failures-they’re individual sensitivities. If you get a rash or stomach upset after switching, it’s worth checking with your pharmacist.
What are narrow therapeutic index drugs, and why are they special?
These are drugs where even a small change in blood level can cause serious harm-like warfarin (blood thinner), digoxin (heart medication), or phenytoin (seizure control). For these, the FDA requires tighter bioequivalence limits: 90-111% instead of 80-125%. Pharmacists are also required to notify prescribers before substituting these generics, and many doctors prefer to keep patients on the same manufacturer.
How do I know if my generic drug is approved and safe?
Check the FDA’s Orange Book online. Search your drug name and look for the therapeutic equivalence code. ‘A’ means it’s approved as equivalent. ‘B’ means there are potential concerns. You can also ask your pharmacist for the manufacturer name and lot number. All approved generics must meet the same strict standards, regardless of who makes them.
Is it safe to switch between different generic manufacturers?
Yes, as long as each version has an ‘A’ rating. Each manufacturer must prove bioequivalence independently. But if you’re on a narrow therapeutic index drug, sticking with the same manufacturer can reduce variability. Talk to your doctor if you notice changes after switching manufacturers.
Final Takeaway
Generic drugs aren’t second-rate. They’re scientifically identical in how they work inside your body. The absorption rates are monitored, tested, and regulated with more precision than most consumer products. The system isn’t perfect-but it’s reliable. And for millions of people, it’s the only reason they can afford to stay healthy.

steve ker
January 10, 2026 AT 15:47Audu ikhlas
January 10, 2026 AT 19:04Sonal Guha
January 11, 2026 AT 12:01TiM Vince
January 12, 2026 AT 08:24Bryan Wolfe
January 13, 2026 AT 06:28Alice Elanora Shepherd
January 14, 2026 AT 18:29beth cordell
January 15, 2026 AT 09:25Cassie Widders
January 16, 2026 AT 21:18Darryl Perry
January 17, 2026 AT 13:15Jose Mecanico
January 19, 2026 AT 12:21jordan shiyangeni
January 20, 2026 AT 16:12